Femtosecond Laser Treatment of Cartilage
Our femtosecond laser studies are conducted in collaboration with the Vukelic Group of Prof. Sinisa Vukelic in the Mechanical Engineering Department of Columbia University. Through combination of the laser technology and the MBL’s expertise in cartilage mechanics, a novel methodology for the induction of crosslinks (CxL) in cartilage through femtosecond laser treatment is being developed.
Biological Basis
The extracellular matrix (ECM) is the primary structural component of cartilage tissue and contains the collagen (COL) fibril network and the proteoglycans (PGs). The COL fibril network is maintained with crosslinks (CxLs) and provides cartilage tensile strength while counteracting the swelling pressure of PGs. The contribution of crosslinking to tensile strength and stability of cartilage COL matrix has been extensively demonstrated in tissue engineering studies [1].Correspondingly, disruption of CxLs results in increased water content and swelling due to the osmotic pressure of PGs, and loss of compressive stiffness in the tissue. Along with swelling, increased proteolytic activity shifts the ECM homeostasis towards degradation of matrix components, progressively leading to cartilage degeneration and loss of function [2]. Thus, induction of cross-links to restore the COL fibril matrix may impede OA progression and repair damage to the bulk tissue.
Laser Theory
The induction of CxLs may be achieved with a non-invasive low-level femtosecond laser. Our collaborator, Sinisa Vukelic has previously demonstrated its effectiveness on corneal tissue [3, 4]. Corneal stroma mainly consists of highly organized type I collagen. Our study aims to extend the use of femtosecond laser technology onto the more complex organization of articular cartilage (Fig 5).
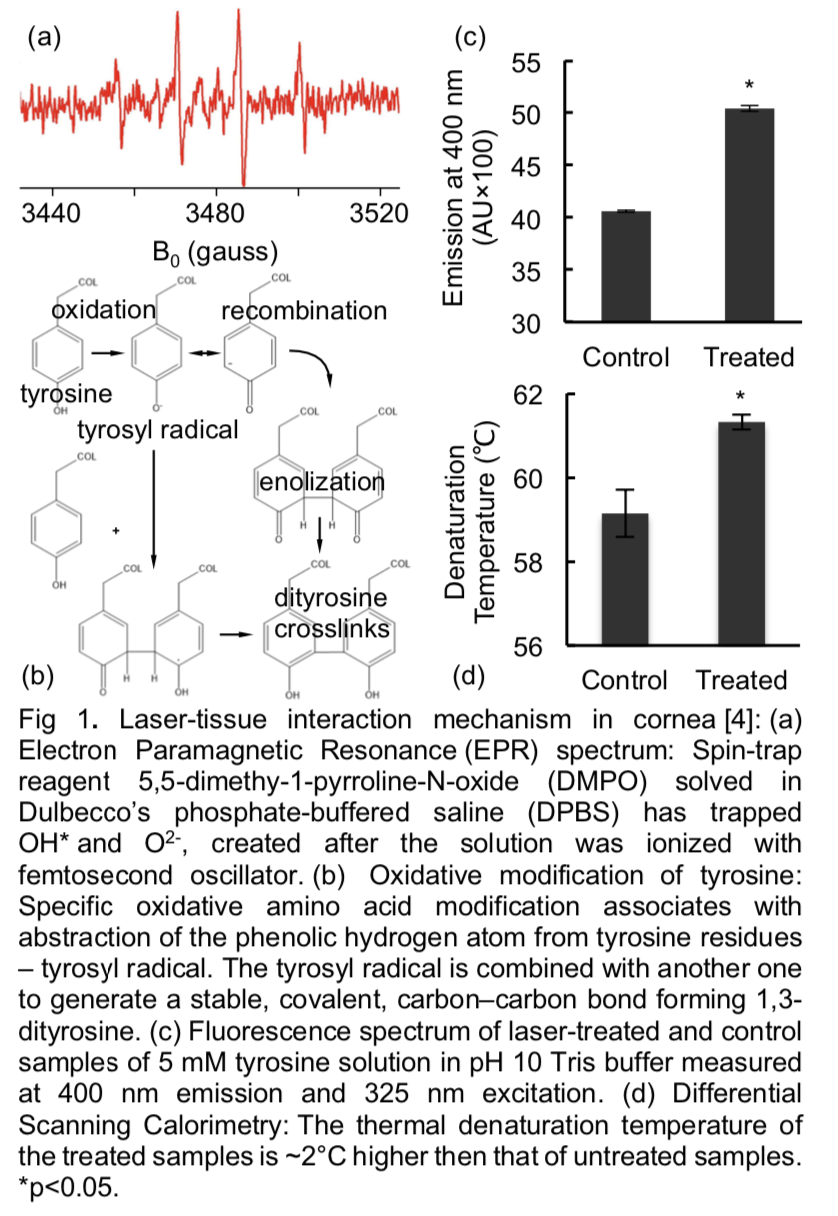
The low-level pulse energy of the laser is below the optical breakdown threshold and thus is able to mimic the effect achieved with higher laser power without damage to surrounding tissue. When femtosecond pulses carrying nano-joule (nJ) energy are focused onto collagen- rich biological media, the interaction results in the formation of a low density plasma (LDP) within the focal volume and its immediate vicinity. We propose to use LDP to generate an ionization field in biological media, without producing tissue-damaging thermoacoustic [5] and shock waves [6]. The ionization field locally ionizes and dissociates interstitial water, creating reactive oxygen species (ROS) (Fig 6a) which initiate photo-oxidation of proteins to form CxL (Fig 1b, c, d), giving rise to spatially resolved alterations in mechanical properties [7]. Our preliminary work has demonstrated the efficacy of femtosecond laser treatment for cartilage CxL induction and the corresponding enhancement of tissue mechanical properties [8].
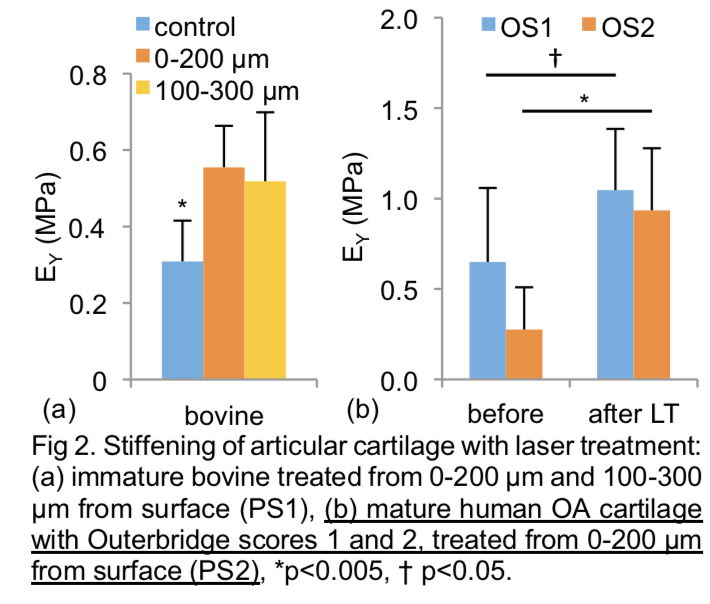
Enhancement of Mechanical Stiffness
We have performed several preliminary studies of cartilage laser treatment with the femtosecond laser that have enhanced the tissue mechanical properties. Studies were conducted on both immature bovine articular cartilage as well as devitalized fibrillated human articular cartilage from different grades of osteoarthritic human cartilage. Results for bovine cartilage treatment showed that femtosecond laser treatment of cartilage tissue enhanced the modulus, EY, by nearly 100%, producing a significantly higher EY than the control group. Additionally, we found that laser treatment significantly enhanced EY for the human osteoarthritic samples, establishing that this laser treatment protocol is equally effective for cartilage from different species and from different grades of osteoarthritic human cartilage. Figure (2) devitalized explants.
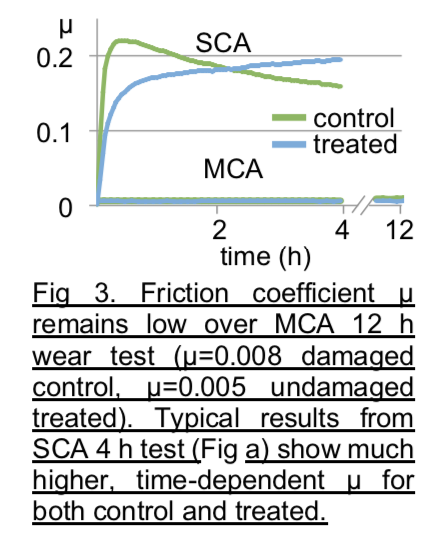
Mechanical Wear Properties
In addition to enhancing the modulus EY, the femtosecond laser treatment has the potential to improve wear resistant properties of tissue. In our studies, we examined the wear resistance of control and laser-treated devitalized immature bovine explants. For control samples, EY decreased significantly with wear testing, exhibiting clear visual evidence of damage (Fig 4). In contrast, laser-treated samples exhibited no significant difference in EY and showed little visual evidence of damage (Fig 4). These results suggest that the most important functional properties of cartilage (sustaining compressive load and producing low wear) are significantly enhanced by our novel laser treatment methodology, without detriment to the friction coefficient.
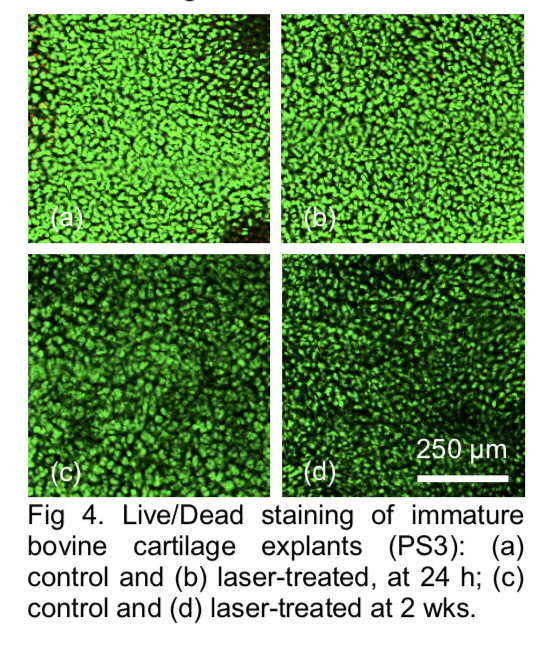
Cell Viability
To evaluate the effectiveness of the laser treatment in a clinical setting, we laser-treated live immature bovine cartilage explants and examined cell viability (Live/Dead Assay Kit, Molecular Probes) 24 h after laser treatment. Confocal images of explants demonstrated no evidence of viability loss from laser treatment. For additional samples maintained for two weeks in culture after laser treatment, cell viability was similarly maintained when compared to untreated controls These results demonstrate that laser treatment does not compromise cell viability for up to two weeks, while achieving equally effective enhancement of the tissue compressive modulus as with devitalized explants.
[1] Asanbaeva A, Masuda K, Thonar E-M, Klisch SM, Sah RL. Cartilage growth and remodeling: modulation of balance between proteoglycan and collagen network in vitro with β-aminopropionitrile. Osteoarthritis and cartilage 2008; 16: 1-11
[2] Dijkgraaf, L et al., J. Oral Maxillofac. Surg., 53(8):924–29, 1995.
[3] Wang C, Fomovsky M, Miao G, Zyablitskaya M, Vukelic S. Femtosecond laser crosslinking of the cornea for noninvasive vision correction. Nature Photonics 2018; in press
[4] Wang C, Fomovsky M, Hall JR, Paik DC, Trokel SL, Vukelic S. A New Paradigm for Use of Ultrafast Lasers in Ophthalmology for Enhancement of Corneal Mechanical Properties and Permanent Correction of Refractive Errors. In: Proc. of SPIE Vol, vol. 10066 2017:100660Y-100661.
[5] Shen N, Datta D, Schaffer CB, LeDuc P, Ingber DE, Mazur E. Ablation of cytoskeletal filaments and mitochondria in live cells using a femtosecond laser nanoscissor. Mech. Chem. Biosyst 2005; 2: 17-25
[6] Schaffer CB, Jamison AO, Mazur E. Morphology of femtosecond laser-induced structural changes in bulk transparent materials. Applied Physics Letters 2004; 84: 1441-1443
[7] Meek KM, Hayes S. Corneal cross‐linking‒a review. Ophthalmic and Physiological Optics 2013; 33: 78-93
[8] Durney KM, Oungoulian SR, Jones BK, Suh JT, Hung CT, Ateshian GA. Cartilage wear initiated by fatigue damage under physiologic loading when fluid load support and boundary lubrication are compromised. In: 2015 Summer Biomechanics, Bioengineering, and Biotransport Conference. Snowbird, UT 2015:SB3C2015-1160.
